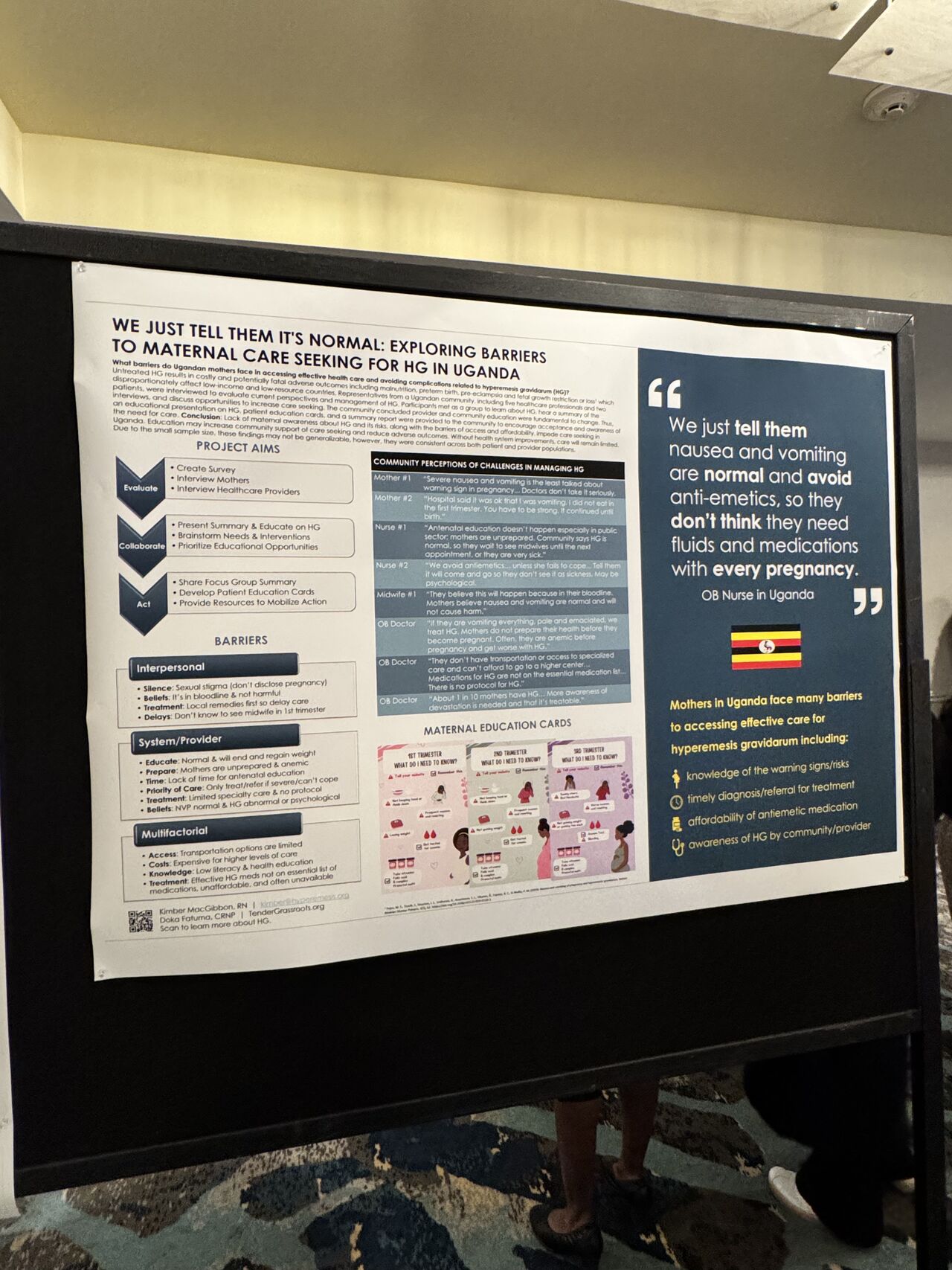
At Usawa Health Initiative, we are committed to advancing maternal health in Uganda through research, advocacy, and innovative healthcare solutions. We are proud to share that our co-founder Dr. Kizza Blair recently had the honor of presenting original research at the International Colloquium on Hyperemesis Gravidarum (ICHG) 2024 held in Ventura, CA, USA, on November 6-7, 2024.. This prestigious event brings together global experts to discuss advancements in understanding and managing Hyperemesis Gravidarum (HG), a severe form of pregnancy-related nausea and vomiting.
Addressing Hyperemesis Gravidarum in Uganda: A Comparative Study
Hyperemesis Gravidarum remains a critical challenge for maternal health in Uganda, where limited treatment guidelines and scarce healthcare resources complicate effective management. Our co-founder’s study aimed to evaluate the efficacy, safety, and cost-effectiveness of two commonly used medications—Promethazine and Ondansetron—to determine the best approach for treating HG in a low-resource setting.
Study Design & Key Findings
The randomized controlled trial included 102 primigravid women (first-time pregnancies), aged 18 to 40 years, diagnosed with HG. Participants were randomly assigned to receive either Promethazine (25 mg every 6 hours) or Ondansetron (4 mg every 8 hours). The primary outcome was the reduction in nausea and vomiting severity, assessed using the Pregnancy-Unique Quantification of Emesis (PUQE) score.
Results Summary:
- Reduction in PUQE Scores:
- Ondansetron: 12.5-point reduction (95% CI: 10.8–14.2)
- Promethazine: 8.3-point reduction (95% CI: 6.9–9.7)
(p<0.01, indicating significant improvement with Ondansetron)
- Maternal Weight Gain:
- Ondansetron: 3.2 kg (95% CI: 2.8–3.6)
- Promethazine: 2.8 kg (95% CI: 2.4–3.2)
(p=0.15, no significant difference)
- Gestational Age at Delivery:
- Ondansetron: 39.2 weeks (95% CI: 38.8–39.6)
- Promethazine: 37.5 weeks (95% CI: 37.1–37.9)
(p=0.02, favoring Ondansetron)
- Neonatal Birth Weight:
- Ondansetron: 3.2 kg (95% CI: 3.0–3.4)
- Promethazine: 2.8 kg (95% CI: 2.6–3.0)
(p=0.03, favoring Ondansetron)
- Adverse Effects:
- Ondansetron: 4%
- Promethazine: 12%
(p=0.03, indicating fewer side effects with Ondansetron)
Key Takeaways & Policy Implications
The findings underscore that Ondansetron significantly outperformed Promethazine in symptom relief, had fewer adverse effects, and was associated with improved maternal and neonatal outcomes. Despite being more expensive, its superior effectiveness suggests it should be prioritized in treatment guidelines for Hyperemesis Gravidarum in Uganda.
However, affordability and accessibility remain critical concerns. While Promethazine is more widely available and cost-effective, its higher rate of side effects may limit patient adherence and overall treatment success. This highlights the urgent need for policy adjustments and increased resource allocation to improve maternal healthcare access in Uganda.
Gratitude & Next Steps
We extend our deepest appreciation to HER Foundation, Bikkja Trust Fund, and ICHG for their generous support, which made this research presentation possible. The insights gained from ICHG 2024 will help inform better treatment strategies and advocacy efforts to improve maternal health outcomes in Uganda and other low-resource settings.
As Usawa Health Initiative continues its mission, we remain dedicated to evidence-based interventions, capacity-building for healthcare workers, and policy advocacy to ensure every mother receives the care she deserves.
Stay connected with us for more updates on our research and impact!